Kasık Fıtığı

LICHTENSTEIN
DynaMesh®-LICHTENSTEIN ve DynaMesh®-LICHTENSTEIN görünür implantlar, kasık fıtıklarının cerrahi tedavisi için tasarlanmıştır ve kasık bölgesindeki fıtık defekti alanındaki yumuşak dokuyu kalıcı olarak köprülemek ve güçlendirmek için kullanılır.
Paylaş
For example: inguinal hernia, left side
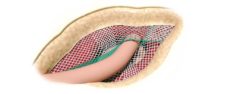

Varying Pore Size
The devices have areas with different pore sizes.
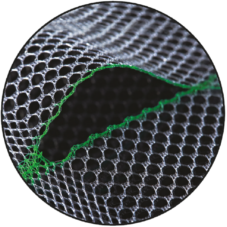
Prefabricated Slit & Smooth Warp-Knitted Selvedges
The devices have a prefabricated slit with smooth warp-knitted selvedges, and they have tear propagation resistance [TR82].

Visibility & Handling
The colouring provides better intraoperative visibility and handling of the device.
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |
When selecting the mesh size, ensure sufficient overlap!
| DynaMesh®-LICHTENSTEIN | 06 cm x 11 cm | PV110611F1/F3/F5/F10 |
| DynaMesh®-LICHTENSTEIN | 7.5 cm x 15 cm | PV110715F1/F3/F5/F10 |
| DynaMesh®-LICHTENSTEIN | 10 cm x 15 cm | PV111015F1/F3/F5 |
| DynaMesh®-LICHTENSTEIN visible | 06 cm x 11 cm | PV170611F3/F10 |
| DynaMesh®-LICHTENSTEIN visible | 7.5 cm x 15 cm | PV170715F1/F3/F10 |
| FX = X unit(s)/box (e.g. F3 = 3 unit(s)/box) |
| Product | DynaMesh®-LICHTENSTEIN (1) DynaMesh®-LICHTENSTEIN visible (2) |
| Surgical Treatment | Inguinal Hernias |
| Surgical Approach | Open |
| Surgical Technique | Lichtenstein |
| Mesh Position | Onlay (Anterior) |
| Fixation | Sutures / Tacks / Tissue Adhesives* |
| Coloured Filaments | Green (1) / Black (2) |
| Smooth Warp-Knitted Selvedges |
|
| Visible Technology |
|
| Materials | – Polyvinylidene fluoride (PVDF) (CAS 24937-79-9) > 99% (w/w) (1) (2) – Phthalocyanine green (CAS 1328-53-6) < 1% (w/w) (1) – Triiron tetraoxide (CAS 1317-61-9) < 1% (w/w) (2) |
| Polymer (Monofilament) | PVDF |
| Biocompatibility |
|
| Ageing Resistance |
|
| Tear Propagation Resistance |
|
| Effective Porosity |
|
| Klinge’s Mesh Classification | Class 1a [102P, TR83] |
| * Tissue adhesive can be used for fixation for direct or indirect inguinal hernias with a defect size of 1.5 cm to 3 cm (European Hernia Society Classification: M2 or L2). |
| Applies to all product sizes | |
| Does not apply | |
| [#] | Reference “#” (see “References”) |
| [TR#] | Internal test report (see “internal test report references”) |
| Limitations | “A” animal trial, “B” bench test, “VIT” in-vitro trial, “P” published results based on the analysis of human mesh explants, “PB” published results mainly based on bench tests |





